The Coulumb or electrostatic force, that is the force of repulsion or attraction between two electric charges, follows the law
where ![]() and
and ![]() are the charges of the two particles,
are the charges of the two particles, ![]() is a fundamental constant, and
is a fundamental constant, and ![]() is the distance between the particles. You may therefore have heard it referred to as the inverse-square law, because of the factor of
is the distance between the particles. You may therefore have heard it referred to as the inverse-square law, because of the factor of ![]() . In quantum mechanics, it is more convenient to deal with energies than forces, so we can instead express this in the form
. In quantum mechanics, it is more convenient to deal with energies than forces, so we can instead express this in the form
or
where ![]() is the electric potential, meaning the energy to insert a charge
is the electric potential, meaning the energy to insert a charge ![]() at a point where the potential is
at a point where the potential is ![]() is
is ![]() . This itself follows from Maxwell’s Equations, which in the case where everything is stationary, imply
. This itself follows from Maxwell’s Equations, which in the case where everything is stationary, imply
which has ![]() as a solution given the charge distribution
as a solution given the charge distribution ![]() .
.
In 4D however, the solution for the electric field produced by a point charge changes to
meaning that the energy scales as the inverse square of distance and force as the inverse cube. This is analogous to the spread of radiation as distance increases. In 3D, its intensity decreases as ![]() because the same amount of radiation is spread out over a larger area at greater distances, and the area of a sphere is
because the same amount of radiation is spread out over a larger area at greater distances, and the area of a sphere is ![]() , but the surface area of a 4D hypersphere increases more quickly as
, but the surface area of a 4D hypersphere increases more quickly as ![]() .
.
It would be possible to just artificially impose an inverse square law instead, but this would be very unnatural. It would be much more difficult to justify that as the result of a more fundamental local law, and it would also mean that many neat properties of electromagnetism would no longer hold. For example, with the inverse square law in 3D and inverse cube law in 4D, a uniformly charged sphere produces an electric field that is constant on the inside and identical to that of a point charge on the outside, and a static electric field cannot penetrate a grounded conductor. Neither of these would be the case for an inverse square law in 4D. Analogous effects would make the screening of nuclear charge by inner electrons behave oddly, although that would hardly be the most unusual consequence of taking chemistry to 4D.
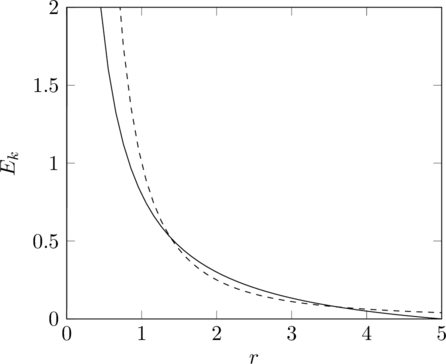
Apart from its natural compatibility with 3D space, an inverse square law force also has the advantage of stable elliptical orbits. With an inverse cube law force, anything in orbit that is getting nearer to the central attractor will tend to continue spiralling in until it collides, and conversely, and object that is getting farther away will tend to continue spiralling off to an infinite distance. This can be seen from the relation of angular momentum to kinetic energy. The kinetic energy of a particle travelling horizontally (i.e. neither towards nor away from the centre) is
And for a particle that is not travelling horizontally, this is a lower bound, since there is kinetic energy in the vertical direction too.
Given that the total energy is constant, this gives an upper and lower bound to the possible radii.
The solid line in this graph represents the difference between the total energy (in this case ![]() ) and the potential energy, which scales as
) and the potential energy, which scales as ![]() , and the dashed line represents the bound on the kinetic energy given above. Within the radius range of approximately
, and the dashed line represents the bound on the kinetic energy given above. Within the radius range of approximately ![]() to
to ![]() , the inequality is satisfied, and at either end of this range, it is possible for the particle to be travelling horizontally, therefore it travels back and forth between these extremes and cannot reach infinity or the centre.
, the inequality is satisfied, and at either end of this range, it is possible for the particle to be travelling horizontally, therefore it travels back and forth between these extremes and cannot reach infinity or the centre.
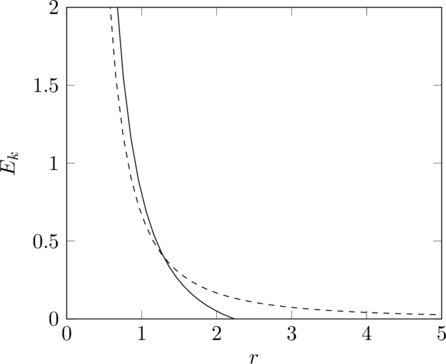
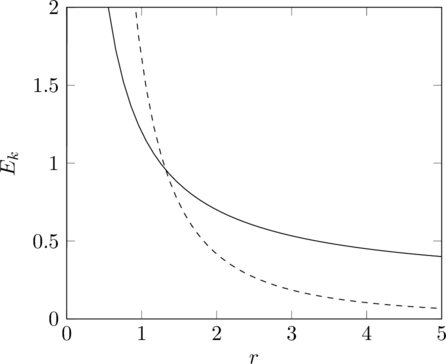
If we replace the potential energy with ![]() though, at least one of the intersections must disappear.
though, at least one of the intersections must disappear.
In the first case, the inequality is satisfied for any ![]() and it is only an equality for
and it is only an equality for ![]() , therefore if the particle is moving outwards, it must turn around at
, therefore if the particle is moving outwards, it must turn around at ![]() and head inwards, then it cannot turn around again because that would require it to momentarily travel horizontally, which it can only do where the graphs intersect, so it continues to spiral in until it hits the central attractor. In the second case, it’s the reverse. A particle travelling outwards cannot turn around, so it eventually escapes the potential well entirely. There are some other cases with different combinations of angular momentum and total energy, but the only case where a stable orbit is possible is when the total energy is
and head inwards, then it cannot turn around again because that would require it to momentarily travel horizontally, which it can only do where the graphs intersect, so it continues to spiral in until it hits the central attractor. In the second case, it’s the reverse. A particle travelling outwards cannot turn around, so it eventually escapes the potential well entirely. There are some other cases with different combinations of angular momentum and total energy, but the only case where a stable orbit is possible is when the total energy is ![]() and the angular momentum is precisely matched to give a circular orbit, but even in this case it is not exactly stable, since any tiny perturbation will cause it to leave orbit one way or the other.
and the angular momentum is precisely matched to give a circular orbit, but even in this case it is not exactly stable, since any tiny perturbation will cause it to leave orbit one way or the other.
The quantum case is similar, although the details are slightly different. Suppose there’s an electron orbiting a nucleus in 3D space. Schrödinger’s equation for this case (omitting some irrelevant constants) is
If the wavefunction is very concentrated around the nucleus, with length scale around ![]() , the (kinetic energy) term with the second derivative
, the (kinetic energy) term with the second derivative ![]() becomes much larger than the Coulumb force term
becomes much larger than the Coulumb force term ![]() since they scale like
since they scale like ![]() and
and ![]() respectively. Since the kinetic term is dominant, we can approximate Schrödinger’s equation as
respectively. Since the kinetic term is dominant, we can approximate Schrödinger’s equation as ![]() , which is a wave equation whose solutions tend to spread out over time unless they’re already plane waves, which suggests that highly concentrated wave functions will tend to spread out under the full equation as well. Conversely, for a very smooth and spread out wavefunction, the
, which is a wave equation whose solutions tend to spread out over time unless they’re already plane waves, which suggests that highly concentrated wave functions will tend to spread out under the full equation as well. Conversely, for a very smooth and spread out wavefunction, the ![]() term dominates. The explanation for why this leads to the electron moving inwards is a bit less direct, since it requires the Coulumb term creating inwards momentum then the kinetic term turning that into motion, but the final result is that there is some intermediate size where the wavefunction is stable.
term dominates. The explanation for why this leads to the electron moving inwards is a bit less direct, since it requires the Coulumb term creating inwards momentum then the kinetic term turning that into motion, but the final result is that there is some intermediate size where the wavefunction is stable.
It can be a bit more intuitive to think of this using the time-independent form of Schrödinger’s equation
which applies in the case of static states of definite energy ![]() . The most stable states are the ones with lowest energy. The
. The most stable states are the ones with lowest energy. The ![]() term is always negative in energy and it is larger in magnitude as the electron gets closer to the nucleus, so while it is dominant (i.e. the wavefunction is smooth and spread out), the energy gets lower the closer-in the electron gets, but at some point the kinetic term becomes larger, and for states where
term is always negative in energy and it is larger in magnitude as the electron gets closer to the nucleus, so while it is dominant (i.e. the wavefunction is smooth and spread out), the energy gets lower the closer-in the electron gets, but at some point the kinetic term becomes larger, and for states where ![]() tends to
tends to ![]() as
as ![]() tends to
tends to ![]() , or at least remains bounded (the physically realistic states), the kinetic term is always positive in energy. There is therefore some minimum in energy at an intermediate size.
, or at least remains bounded (the physically realistic states), the kinetic term is always positive in energy. There is therefore some minimum in energy at an intermediate size.
Now consider the 4D case. With the change from inverse-square law attraction to inverse-cube law, the equation becomes
Suppose some ![]() is a solution to this, then so is
is a solution to this, then so is ![]() , where
, where ![]() is an arbitrary scale factor, since every term in the equation scales by a factor of
is an arbitrary scale factor, since every term in the equation scales by a factor of ![]() . This means that if there is some stable atomic state of a certain size and energy, there is another similar state of half the size and four times the energy. Since the energy of a stable state is negative, this smaller state is lower in energy and the atom would tend to release energy and fall into the lower state, but there are always still lower-energy states, and so it would just keep releasing energy until one of the approximations involved breaks down, with either relativistic effects or the non-zero radius of the nucleus becoming relevant. If the radius of the nucles becomes relevant, then chemistry degenerates into something rather like the plum pudding model, where the electrons are embedded within the nucleus and adjacent nuclei are likely to physically touch each other, rather than having a diffuse cloud of electrons around an essentially point-like nucleus. If relativistic effects come into play, I’m not even sure it wouldn’t just make the instability worse. I haven’t actually checked.
. This means that if there is some stable atomic state of a certain size and energy, there is another similar state of half the size and four times the energy. Since the energy of a stable state is negative, this smaller state is lower in energy and the atom would tend to release energy and fall into the lower state, but there are always still lower-energy states, and so it would just keep releasing energy until one of the approximations involved breaks down, with either relativistic effects or the non-zero radius of the nucleus becoming relevant. If the radius of the nucles becomes relevant, then chemistry degenerates into something rather like the plum pudding model, where the electrons are embedded within the nucleus and adjacent nuclei are likely to physically touch each other, rather than having a diffuse cloud of electrons around an essentially point-like nucleus. If relativistic effects come into play, I’m not even sure it wouldn’t just make the instability worse. I haven’t actually checked.
In order for there to be interesting chemistry to study some solution must be found to the fact that every possible state is unstable. I would prefer to avoid both the plum pudding and the relativistic cases, the first because it feels inelegant, too dissimilar to real-life chemistry, and difficult to simulate, and the second mostly just because it would be hard to simulate and I expect it wouldn’t work anyway, though it might be interesting to consider at some point. I will therefore be assuming from here on that there is an additional short-range repulsive force which stops the electrons from falling into the nucleus. It is of the form
since that seems the most physically plausible (for a force mediated by a massive vector boson with ![]() proportional to its mass). It would also make sense for this to cause electrons to repel each other, but I’ll be ignoring this for simplicity (perhaps the nucleus’s charge is very large and the electron’s is very small). It would also cause nuclei to repel each other, but in this case I’m actually justified in ignoring it, because the distances between nuclei in normal molecules will turn out to be far greater than the range of this force anyway. I call it the s-force, because the closest I could think of to an antonym for “plum pudding” was “slimming”.
proportional to its mass). It would also make sense for this to cause electrons to repel each other, but I’ll be ignoring this for simplicity (perhaps the nucleus’s charge is very large and the electron’s is very small). It would also cause nuclei to repel each other, but in this case I’m actually justified in ignoring it, because the distances between nuclei in normal molecules will turn out to be far greater than the range of this force anyway. I call it the s-force, because the closest I could think of to an antonym for “plum pudding” was “slimming”.
Although the s-force sets a lower bound on the sizes of orbitals, the fact that there is no upper bound turns out to still be important. In real life, the smallest atoms (hydrogen) and the largest (caesium) differ in size by only about a factor of 10. This is because the balance between nuclear charge and kinetic energy sets the overall scale of the orbitals, and the effective nuclear charge experienced by the outermost electrons is always within an order of magnitude or so of 1. As more electrons are added, the innermost orbitals shrink and more appear above them, keeping roughly in balance overall. In my simulations of 4D, the size of each orbital is roughly set by the one below it in an exponentially increasing chain, with the innermost having a constant size set by the s-force, therefore the largest atoms can be huge. I vaguely remember there being a difference in scale of tens of thousands, but checking my actual notes the biggest difference I can find is about a factor of 100. I may be remembering a different set of parameters than I ended up actually going with. This is still a factor of ![]() in volume and
in volume and ![]() in energy though, leading to some elements with peculiar properties.
in energy though, leading to some elements with peculiar properties.
The largest most diffuse atoms are somewhat analogous to alkali metals, with the outermost shell having only an s orbital filled, but their properties are much more extreme. With ionization energies ten thousand times smaller than normal, there is almost no situation in which they would appear in unionized elemental form. If they were somehow isolated, they could form stable plasmas at room temperature, but of course this gas would be able to reduce practically anything it came in contact with. At sufficiently cold temperatures for them to freeze, they would probably form metals of extremely low densities, millions of times lighter than a normal solid. As any optical photon would easily cause electrons to escape from such a metal, it would probably have peculiar optical properties, although I’m not sure what those would be. Certain real-life metals such as gold and caesium have a yellow appearance because their plasma oscillations have low enough energy to absorb blue light. For 4D alkali metals, these would be even lower energy, perhaps giving the metals a deep red or even black colour. Most of these properties are just somewhat informed guesses though, as the only actual simulations of these elements I’ve been able to do so far are only of isolated atoms.
Leave a Reply